| Vocademy |
Batteries
 |
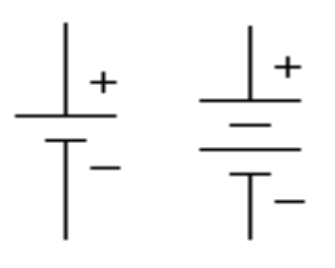 Schematic symbol |
So far, we have treated batteries as if they were perfect voltage sources. That is, we have assumed that a battery will produce the same voltage under all circumstances and has no other effects on a circuit. In reality, batteries have their own characteristics and batteries with different chemistry have different characteristics.
Terminology
What we usually call a battery should be called a voltaic cell or galvanic cell. A "battery" is technically a group of voltaic cells. However, it has become common to refer to a single voltaic cell as a battery and a group of voltaic cells "batteries".How batteries are constructed

| Carbon-zinc voltaic cell |
A battery is constructed by combining two dissimilar metals with an electrolyte (an acid or base) that chemically reacts with the metals. The unequal chemical action causes an imbalance of free electrons and therefore an electric potential between the electrodes of the cell. Different metal / electrolyte combinations will result in different voltages as well as differences in other characteristics. For example, a Carbon-Zinc cell produces 1.6 volts. The voltage appears to drop off steadily as the battery is used. A mercury-oxide cell produces 1.35 volts and the voltage remains constant through most of the life of the battery.
Internal resistance
Internal resistance is a theoretical limitation of how much current a battery can deliver. As stated above, a carbon-zinc cell appears to lose voltage over time. In theory this is because the internal resistance increases as the battery is used. Internal resistance is calculated by dividing the open circuit voltage by the closed circuit current. This is the same procedure for determining the Thevenin equivalent impedance of a circuit (see Thevenin's Theorem).
 |
| Equivalent circuit for a battery |
The internal resistance of a battery is the same concept as output impedance with other circuits (Thevenin equivalent impedance, output impedance and internal resistance are different names for the same thing). If you try the above method to determine the internal resistance of a battery (measuring the open circuit voltage and the short circuit current), be sure to have the current meter across the battery for as short a time possible. Shorting batteries for too long can cause fire or cause the battery to explode.
Batteries and EMF
Earlier (see What is Voltage), we discussed the difference between EMF and voltage. Recall that EMF is a force that tends to move electricity, and voltage is an electrical pressure differential that develops where the flow of electrical current is blocked. The standard battery model (shown above as the equivalent circuit for a battery) shows a source of EMF (represented by a battery symbol) and a resistance in series with that EMF source. The voltage across the terminals of a battery is usually assumed to equal the total EMF. This assumes no current flow since a voltmeter has a very high impedance. If there is current flow, the voltage at the terminals will be the total EMF minus the voltage differential across the internal resistance. This concept has been discussed previously under Ohm's Law and Thevenin's Theorem, Output Impedance and Input Impedance.
Applications and Safety
The terminals of batteries should never be shorted together except for short periods in order to test the battery. Some shorted batteries, particularly alkaline batteries, will explode.Fresh batteries should never be mixed with old batteries. Fresh batteries will force a reverse charge on old batteries near exhaustion. This is likely to cause the old batteries to leak.
Some batteries contain powerful acid (lead-acid batteries for example). Others contain other hazardous materials such as mercury.
Batteries that are not designed to be recharged should never be recharged. For example, standard alkaline batteries are prone to leak if recharged. However, rechargeable alkaline batteries are available and labeled as such.
Touching Batteries - Answers to Questions
| Vocademy |