| Vocademy |
What is Voltage
Voltage is like air pressure
Voltage is analogous to air pressure. Air molecules repel each other. The reason why isn't important, but the fact is that if you squeeze a volume of air into a smaller space, the air molecules will exert a greater pressure on each other and the inner walls of the container. It's like filling a tire. If you force air into a tire, you will have some air pressure inside the tire. Force more air in, and you will get more pressure. Force enough air into the tire, and you will get enough pressure exerted on the tire walls to support the car. Once the tire is inflated, if you open the filler valve, the high-pressure air inside the tire will escape to the atmosphere where the pressure is lower.There is an electronic component called a capacitor. We will discuss capacitors later. However, a capacitor acts much like a tire. We can put electricity into a capacitor and get electrical pressure. We call electrical pressure voltage. Put in more electricity, and we will get more voltage. We can store electricity under pressure (voltage) in a capacitor just like we can store air under pressure in a tire.
Another way to develop air pressure is to block airflow in a hose. For example, if we open the valve of the air hose used to fill our tire, the air will escape, and there will be little or no pressure in the hose. However, if we block that flow, we will get significant pressure in the hose (like putting your thumb over a garden hose). Likewise, if you block the flow of electricity in a wire, you will get voltage in the wire.
Voltage is always a differential
Whenever we measure pressure, we are always comparing one pressure to
another. Voltage is no different. Let’s look at the tire again. Let’s say
you are at the beach. The location is important because it puts you at sea
level. Now let’s say you put a pressure gauge on the tire and see that it
contains a pressure of 30 pounds per square inch (30 psi). The question is,
what is the actual pressure inside the tire? Isn’t it 30 psi? Actually, no.
It’s about 45 psi. How can that be? Why does the gauge say 30 psi if the
actual pressure is 45 psi? The reason is that the air around you already has
a pressure of about 15 psi (Actually about 14.7 psi, but let’s keep the math
simple). The gauge is actually comparing the pressure inside the tire to the
pressure outside the tire and telling you the difference. If you drive your
car to an altitude of 6,000 feet above sea level, the ambient pressure is
about 12 psi. Assuming nothing changed except the altitude, your gauge will
now read about 33 psi. In Tibet, there are two roads that go higher than
18,000 feet above sea level. At this altitude, where the ambient air
pressure is about 7.5 psi your gauge will read about 37.5 psi. Finally, if
you could get your tire into outer space, where the ambient pressure is
zero, you gauge will finally read the actual pressure in your tire, which is
45 psi.
Measuring voltage is like measuring air pressure
Voltage, being a measurement of pressure, works the same way. This is why a voltmeter has two probes. The red probe will normally go to the higher voltage and the black probe will normally go to the lower voltage. The meter will then tell the voltage difference between the two probes. One voltage is meaningless unless it is compared to another voltage.A barometer tells us the difference between two air pressures
If pressure is always a differential, where do we get the 15 psi for the ambient air pressure? In 1643 Evangelista Torricelli wanted to find out why suction pumps could not pump water higher than about 34 feet. He made a glass tube with an inside diameter that equaled one square inch. The tube was about three feet long and sealed at one end. He filled the tube with mercury and upended it into a bowl of mercury. Mercury flowed out of the tube into the bowl as you would expect, but stopped when the column of mercury in the tube fell to about 30 inches tall.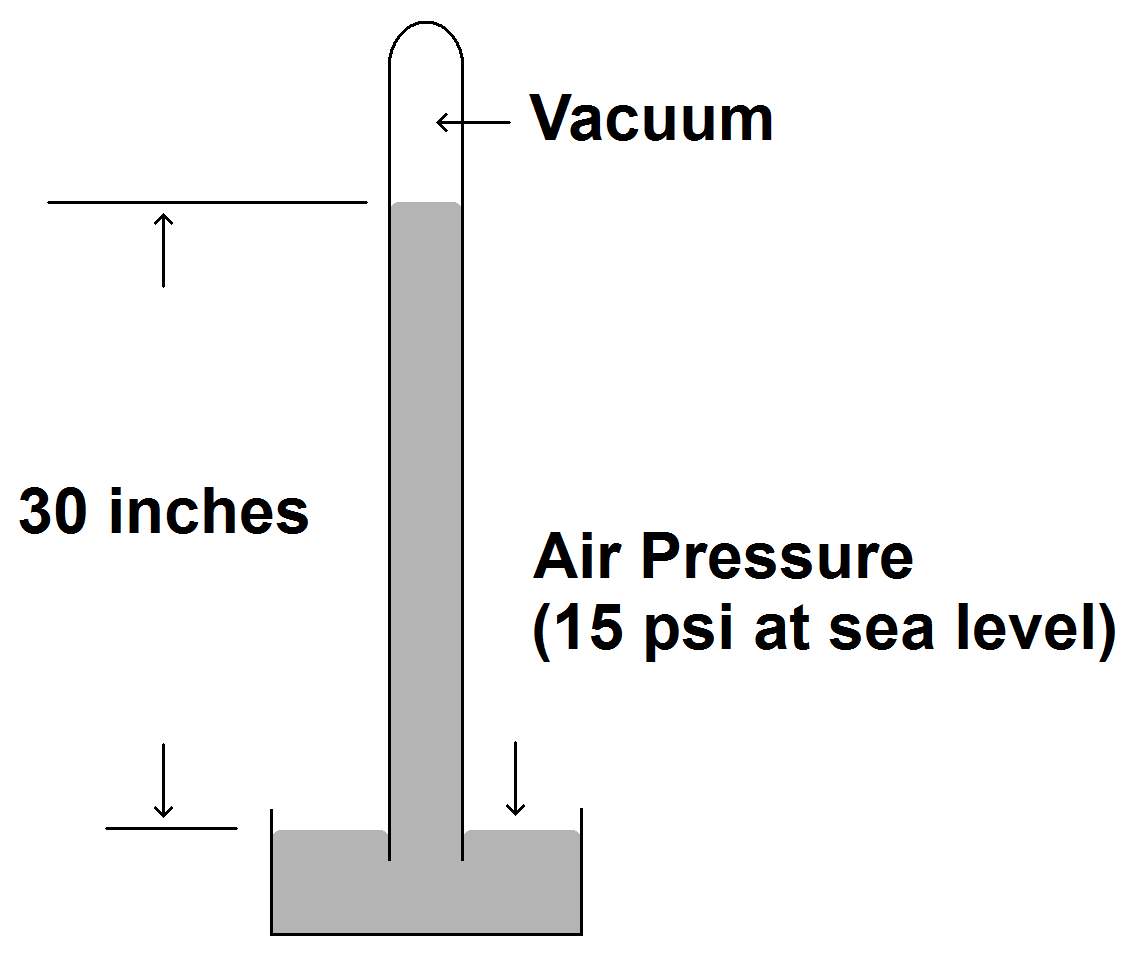
| Torricelli’s barometer. At sea level, the ambient air pressure will push the mercury about 30 inches up the evacuated tube. |
The reason the mercury did not fall below 30 inches was that the air was
pushing down on the surface of the mercury in the bowl. This pressure forced
the mercury up the tube as there was no pressure at the top of the tube to
resist it. No air had been introduced into the tube, so the space above the
column of mercury contained a vacuum. Torricelli then weighed the mercury in
the tube. It weighed about 14.7 pounds. Therefore, the pressure pushing the mercury up the tube was equal to about 14.7 pounds per
square inch.
The height of the mercury in the tube is the same regardless of the size or shape of the tube. Putting a ruler next to the tube gives you the ambient air pressure measured in the convenient units of inches of mercury. This is not a joke. Air pressure may be stated in pounds per square inch, newtons per square meter (pascals) or bars, but in the U.S. is commonly stated in inches of mercury (inHg). Other countries may use millimeters of mercury (mmHg). At sea level, the standard ambient air pressure is 29.92 inHg. Torricelli’s barometer measures ambient air pressure by comparing it to a vacuum. We get one pressure compared to another pressure where that other pressure is absolute zero.
A voltmeter tells us the difference between two voltages
Voltage, being electrical pressure, is measured much like air pressure. A
voltmeter has two electrical probes. When you use a voltmeter, it is telling
you the difference in voltage between those probes. It tells you how much
higher or lower the voltage at the red probe is compared to the voltage at
the black probe.
The height of the mercury in the tube is the same regardless of the size or shape of the tube. Putting a ruler next to the tube gives you the ambient air pressure measured in the convenient units of inches of mercury. This is not a joke. Air pressure may be stated in pounds per square inch, newtons per square meter (pascals) or bars, but in the U.S. is commonly stated in inches of mercury (inHg). Other countries may use millimeters of mercury (mmHg). At sea level, the standard ambient air pressure is 29.92 inHg. Torricelli’s barometer measures ambient air pressure by comparing it to a vacuum. We get one pressure compared to another pressure where that other pressure is absolute zero.
A voltmeter tells us the difference between two voltages
Voltage, being electrical pressure, is measured much like air pressure. A
voltmeter has two electrical probes. When you use a voltmeter, it is telling
you the difference in voltage between those probes. It tells you how much
higher or lower the voltage at the red probe is compared to the voltage at
the black probe.
| A typical voltmeter with its black and red probes. |
If a voltmeter reads 8.2 volts, it is telling you that the voltage at the red
probe is 8.2 volts higher than the voltage at the black probe. If a
voltmeter reads -12.6 volts, it is telling you that the voltage at the red
probe is 12.6 volts lower than the voltage at the black probe. This, of
course, assumes the standard conventional-current model (discussed later)
where positive is greater than negative.
How do you find zero volts?
What is the actual voltage at the black probe? There is no way of knowing. All a voltmeter can tell you is the difference between two voltages. That’s it. There is no “actual” voltage at the black probe to measure. All you can do is tell that some voltage is higher or lower than the voltage at the black probe. An absolute zero voltage has never been defined so there is no electrical equivalent of a Torricelli’s barometer. No device can give you an absolute voltage reading. We will learn what zero volts means in electronics later when we discuss how to measure voltage.Göethe's barometer acts more like a voltmeter than Torricelli’s barometer
The renowned German writer and amateur scientist, Johann Wolfgang Von Göethe,[1] developed a water-based barometer that tells what the ambient air pressure is compared to an arbitrary air pressure (rather than an absolute pressure like Torricelli's barometer).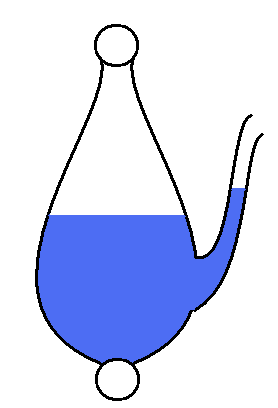
| Göethe’s barometer. Here the ambient air pressure is lower than the air pressure sealed inside the vessel. Get out your umbrella. |
The internal pressure of Göethe's
barometer is whatever the ambient pressure was at the time the
barometer was filled. A voltmeter can only tell you what one voltage
is compared to some other arbitrary voltage. Göethe's barometer can
only tell you how the current ambient air pressure compares to whatever
pressure is sealed inside. It is useful in predicting weather because
storms usually come following a drop in air pressure.
Voltage vs. electromotive force
You may hear that electromotive force is a hifalutin name for voltage. However, although both are measured in volts, voltage and electromotive force are not the same. We will see later that you can have an impetus moving electrons (electromotive force) with no measurable electrical pressure (voltage). Voltage occurs when electromotive force causes electrons to bunch together. This generates electric pressure, much like forcing air molecules to bunch together causes air pressure. We will discuss this further when we cover Ohm's Law, Thevenin's Theorem, and where otherwise appropriate.
Understanding Voltage
Page summary:
- Voltage is like air pressure
- Just like measuring air pressure, when we measure voltage, we always measure the difference between two voltages (one voltage is meaningless unless we compare it to another voltage).
- A voltmeter tells us the voltage difference between the two probes.
- We cannot find an absolute voltage because an absolute zero voltage has never been defined.
—————————
| Vocademy |